Abstract
Epstein-Barr virus associated lymphoproliferative disease is a serious complication of immunosuppression, particularly after transplantation. Initial treatments usually comprise reduction of immunosuppression, rituximab and/or chemotherapy. However, some patients fail to respond or are unsuitable for chemotherapy and so are candidates for adoptive cellular therapy. Here we report outcomes from the use of a bank of 25 third party derived Epstein-Barr virus specific lymphocyte cell lines cryopreserved for immediate use, issued on a best-HLA match basis. Cells have been issued to 70 patients. The infusions were well tolerated, although in two patients, evidence of mild, transient, cutaneous GVHD was observed, both treated with topical agents. In one patient, there was a temporary worsening of neurological symptoms, thought to represent a tumour flare in a patient who responded. 59 patients have received cells with a follow up of >6 months, 34 (58%) male and 25 (42%) female. The median age was 31 years (range 1 - 82). 48 patients had received transplants, 28 HSCT and 20 solid organs. All HSCT patients had received transplants from unrelated donors. SOT types were as follows: kidney, 10; liver, 3; heart, 2; bowel, 1; liver, small bowel and pancreas, 1; combined liver and kidney, 1; combined kidney and pancreas, 1; heart and kidney, 1. Eleven patients were immune suppressed due to causes other than transplantation: five with congenital immunodeficiencies, two on maintenance for acute lymphoblastic leukemia and two on immunosuppressive drugs (1 Crohn disease and 1 Systemic Lupus Erythematosus). One patient had EBV positive natural killer/T cell lymphoma and one diffuse large B cell lymphoma of the elderly.
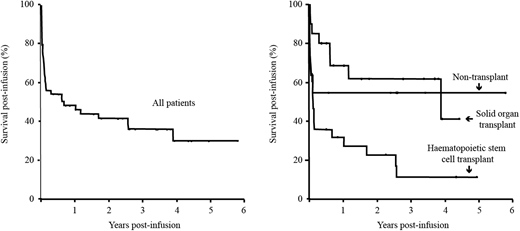
Responses were observed in 35/59 (59%) patients, with rates being highest (75%) post-solid organ transplantation, intermediate (64%) in non-transplant cases and lowest (46%) after hematopoietic stem cell transplantation (p=0.13, Fisher exact). Although most patients were treated after more conventional therapies had failed or were deemed inappropriate, 39% were alive at time of census. Overall survivals were only somewhat less than response rates after solid organ transplantation (60%) and in non-transplant (54%) patients, but worse (18%) after hematopoietic stem cell transplantation (p=0.007, logrank). The mean survival was 2.3 years (95% CI 1.6 - 3.0) and median survival 0.7 years (95%CI 0.0 - 1.8y). Survivals were higher in patients who had not had a transplant or after undergoing SOT versus HSCT, and this difference was statistically significant (p=0.007; log rank). Outcomes were notably good for solitary central nervous system lesions with 12/13 responses (p=0.012, Fisher exact). At census, 132 HLA matching requests have been processed and 61 allocation reviews completed. The median number of class I matches was 3 (range 0-6) and class II matches 2 (range 0-4). The median number of class I+II matches was 4.5 (range 0-9). For CTLs issued the median number of class I matches was 3 (range 1-6) and class II matches 2 (range 0-4). The median number of class I+II matches was 5 (range 2-9). Response rates were moderately higher using better HLA-matched lymphocytes, although this only reached statistical significance (p=0.043) if a one tailed linear-by-linear association test was used. Despite the license covering all European Union countries, locations of recipients were biased towards the United Kingdom (58/64). These data support the use of third party derived cytotoxic lymphocytes in this difficult to treat group of patients, although their current use after hematopoietic stem cell transplantation remains to be optimized.
Kazi:Shire: Other: Vonvendi was provided by Shire. Vickers:GSK: Equity Ownership; University of Aberdeen: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal